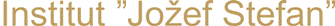RESEARCH
Research programme
Simulations of physico-chemical processes at metal surfaces, such as simple catalytic reactions, at metal surfaces
Within this project we study by means of density-functional-theory calculations some elementary steps in important surface reaction processes based on heterogeneous metallic catalysis. Currently investigated topics are:
Adsorption and dissociation of nitrous oxide (N2O) on Pd(110) and Rh(110) surfaces: N2O is an intermediate in the removal of NOx on automobile three-way catalysts. Its decomposition on rhodium and palladium has attracted much attention, because these metals are good catalysts and N2O is the main byproduct in this process. The emphasis of this study is to understand the mechanism of the peculiar hyperthermal inclined desorption of nitrogen observed recently during thermal dissociation of N2O on above-mentioned (110) surfaces.
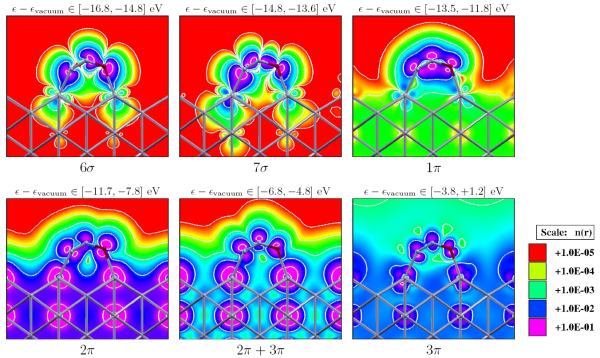
Adsorption of ethylene (C2H4) on silver surfaces: The epoxidation of ethylene, catalyzed by silver, is one of the most important selective oxidation processes based on heterogeneous metallic catalysis. This reaction produces ethylene epoxide (oxirane), which is an important intermediate in the fabrication of glycols and polyols. The emphasis of this study is to understand the influence of surface imperfections (defects) on the adsorption of ethylene. We have found that less coordinated surface atoms located near defects are more reactive, and are therefore the active reaction sites. The role of adsorbed oxygen in the adsorption of the ethylene is also investigated. We have found that atomic oxygen adsorbed on the surface influences the adsorption properties of the surface only slightly, while subsurface atomic oxygen influences the reactivity of the surface to a much larger extent.
Dehydrogenation of methane (CH4) on transition metal surfaces: Methane is a principal component of a natural gas reserves. Therefore it is an important resource for energy and chemicals productions, but due to its gas phase nature it is not very suitable for transportation. It would be desirable, therefore, to transform it to a liquid such as methanol, which would then be easily transportable. The efficiency of transition-metal catalysts to promote these and other related reactions is limited by the tendency of dehydrogenation to proceed until graphite is eventually formed on the surface, thus poisoning the catalyst. The purpose of the study is to investigate the possibility of designing a reaction site in such a way, that the dehydrogenation of methane would be effectively activated, but the dehydrogenation of methyl effectively hindered.